cooling OPEL GT-R 1973 User Guide
[x] Cancel search | Manufacturer: OPEL, Model Year: 1973, Model line: GT-R, Model: OPEL GT-R 1973Pages: 625, PDF Size: 17.22 MB
Page 518 of 625

HEATER SYSTEM. GT9A- 9
REMOVAL AND INSTALLATION HEATER VALVE
Removal
1. Remove lower radiator hose and drain and collect
coolant.
2. Disconnect control cable.
3. Loosen heater hose clamps and remove valve from
hoses. See Figure 9A-17.SPECIFICATIONS
EngineRecommended Coolant
.__,.__...._...__.,.,...,......Thermostat Opens At (Degrees) F.
..__..___...Installation
1. Install valve into heater hoses and install hose
clamps.
2. Connect control cable.
3. Install lower radiator hose and add collected coo-
lant.
.....................................Ethylene-Glycol Base
...................................................................189Cooling
SystemCapacity(WithHeater)..........................................................................6Qt.BlowerMotorType
......................................................................................................12VDC
BlowerFanType
..................................................................................................SquirrelCage
Page 525 of 625

9A-16 1973 OPEL SERVICE MANUAL
Figure 9A-44 Shroud Cover AttachmentsFigure 9A-46 Heater Motor Attaching Screws
Figure 9A-45 Heater Motor Wires
InstallationFigure 9A-47 Sealing Shroud
1. Install heater motor, attaching with three (3)
screws. See Figure 9A-46.
2. Connect multiple plug on left side of shroud. See
Figure 9A-45.3. Seal shroud cover front and rear contacting areas
with sealing cement. See Figure 9A-47.
4. Install shroud cover, attaching with five (5)
screws.SPECIFICATIONS
EngineRecommended Coolant
..........................................................................Ethylene-Glycol Base
ThermostatOpensAt(Degrees)
F.......................................................................................189Cooling System Capacity (With Heater)
..........................................................................6 Qt.
Blower Motor Type
......................................................................................................12 VDC
Blower Fan Type
..............................................................................................................Blade
Numberof FanBlades
..............................................................................................................7
Page 528 of 625
REFRIGERANT COMPONENTS ALL MODELS9s. 19
Figure 98.3 Effect of One B.T.U. on One
Pc’und of
water
teristics of heat if we think of heat as a sort of color-
ing dye. If we add one drop of red dye to a glass of
water, it will turn slightly pink. Another drop will
make the water more reddish in color (Fig.
9B-4).The more drops of dye we add, the redder the water
will get. Each drop of dye corresponds to 1 Btu and
the succeedingly deeper shades of red are like in-
creases in temperature.
Figure
98-4 Addition of B.T.U. Heats Water
It may seem a little puzzling to talk about beat in a
story on air conditioning but, when you stop to
think about it, we are handling heat exclusively. Al-
though we ordinarily think of an air conditioner as
a device for making air cold, it doesn’t do that di-
rectly. What it does is take heat away from the in-
coming air and transfer that heat outside the vehicle.
We know now that cold is nothing more than the
absence of heat, and that heat always flow from a
warm object to a colder one. We also have
:a clearer
idea of how heat is measured.
From everything we’ve learned about heat
EO far, it
seems to behave in a perfectly normal manner. Yetsometimes heat will disappear without leaving a sin-
gle clue.
Ice vs. Water for CoolingEtery once in a while in the old days, the ice-man
would forget to refill the ice-box. Occasionally, as the
last sliver of ice melted away, somebody would come
up with a bright idea. He would remember that the
water in the drain-pan always felt ice-cold when he
had emptied it other times. So, he would get the
thermometer out and check its temperature. Sure
enough, it usually was about as cold as the ice. Why
not put the drain-pan back in the ice compartment
to keep things cold until the iceman returned the
next day
It was a good idea. but it never worked. For some
strange reason the ice-box never stayed cold. The
drain water soon got quite warm and in a couple of
hours, the butter in the ice-box would begin to melt,
the milk would start to sour, and the vegetables
would wilt.
Why did this happen? The drain water was only a
few degrees warmer than the ice yet it didn’t draw
nearly as much heat out of the stored foods. How-
ever, the difference between the behavior of cold
drain water and ice is the real secret as to how any
refrigerator works and we can easily learn the an-
swer by using an ordinary thermometer.
When we put a drain pan full of cold water into the
ice compartment, we expect the heat to flow from the
warm foods to the colder water. Remember, that
heat always flows from a warm object to a colder
object and when we add heat to water, it gets
warmer. Each Btu of heat added to a pound of water
makes it one degree warmer.
Figure 98.5 Melting Ice Remains at 32 Degrees
Page 529 of 625

98-20 1973 OPEL SERVICE MANUAL
If we were to put a thermometer in the cold drain
water, we would see the temperature gradually creep
upwards. That is to be expected because heat is flow-
ing into the cold water making it warmer. Before
long the water would be as warm as the stored foods.
Then the water could no longer attract heat because
heat will not flow from one warm object to another
equally warm object. Since we no longer can draw
heat out of the foods we no longer are cooling them.
Now, let’s see what happens when we put ice instead
of cold water into the ice-box. This time, we’ll set the
thermometer on top of the ice (Fig. 9B-5). When wefirst look at the thermometer, it reads 32 degrees. A
couple of hours later, we open the ice compartment
door. The ice block is smaller because some of the ice
has already melted away
- but the thermometer still
reads 32 degrees. Again, still later, even more of the
ice has melted, yet the termometer continues to read
32 degrees. So long as any ice remains, no matter
how much of it has melted away, the temperature of
the ice stays right at 32 degrees.
All this time the ice has been soaking up heat, yet it
never gets any warmer no matter how much heat it
draws from the stored food. On the other hand, the
cold drain water got progressively warmer as it
soaked up heat. Why is it the addition of heat will
make water warmer yet won’t raise the temperature
of ice above the 32 degrees mark? If we till one
drinking glass with ice and another with cold water,
and put both glasses in the same room where they
could absorb equal amounts of heat from the room
air, we will find it takes much, much longer for the
ice to melt and reach room temperature than it did
for the water in the other glass to reach the same
temperature. Obviously, most of the heat was being
used to melt the ice. But it was the heat that appar-
ently disappeared or went into hiding because if
couldn’t be located with a thermometer. To best de-
scribe this disappearing heat, scientists turned to
Latin for the right word. They chose the word “la-
tent” which means hidden.
Latent Heat
So latent heat is nothing more nor less than hidden
heat which can’t be found with a thermometer.
What happens to the latent heat? Where does it
disappear to? At first it was thought it was in the
water that melted from the ice. But that wasn’t ex-
actly the right answer because, upon checking water
temperature as it melts from ice, it will be found that
it is only a shade warmer than the ice itself. It is not
nearly warm enough to account for all the heat the
ice had absorbed. The only possible answer is that
the latent heat had been used up to change the ice
from a solid into a liquid.
Many substances can be either a solid, or a liquid, ora gas. It just depends on the temperature whether
water for example was a liquid, or a solid (ice), or gas
(steam) (Fig.
9B-6).Figure 99-6 Temperature Determines State of Water
If we put some water in a tea-kettle, set it over a tire
and watch the thermometer as the water gets hotter
and hotter, the mercury will keep rising until the
water starts to boil. Then the mercury seems to stick
at the 212 degrees mark. If we put more wood on the
fire, despite all the increased heat, the mercury will
not budge above the 212 degree mark (Fig.
9B-7).Figure 98.7 Boiling Water Never Exceeds 2 12
DegreesEven though many housewives won’t believe it, no
matter how large or hot you make the flame, you
can’t make water hotter than 2 12 degrees. As a liquid
changes into a gas, it absorbs abnormally great
amounts of heat without getting any hotter. Here is
another instance where heat disappears.
Now we have two different kinds of latent heat,
which are quite alike. To keep their identities sepa-
rate, the first one is called latent heat of fusion. Since
fusion means the same as melting, it is a good de-
scriptive name. The other kind is called latent heat
of vaporization because‘ that means the same as
evaporation.
It may seem as though we have drifted into a story
Page 532 of 625

REFRIGERANT COMPONENTS ALL MODELS96.23Figure 96-l 3 Basic Refrigerant Circuit
we get the heat-laden vapor outside, we can com-
press it with a pump. With enough pressure, we can
squeeze the heat out of “cold” vapor even in a warm
room. An ordinary.radiator will help us get rid of
heat.
By removing the heat, and making the refrigerant
into a liquid, it becomes the same as it was before, So,
we can run another pipe back into the cabinet and
return the refrigerant to the flask to be used over
again.
That is the way most mechanical refrigerators work
today. Now, let’s look at an air conditioning unit to
see how closely it resembles the refrigerator we have
just described.
Basic Air ConditionerWhen we look at an air conditioning unit, we will
always find a set of coils or a tinned radiator core
through which the air to be cooled passes. This is
known as the “evaporator” (Fig.
9B-14). It does the
same job as the flask of refrigerant we
spok.e about
earlier. The refrigerant boils in the evaporator. In
boiling, of course, the refrigerant absorbs heat and
changes into a vapor. By piping this vapor outside
the car we can bodily carry out the heat that caused
its creation.
Once we get vapor out of the evaporator, all we haveFigure 98.14 Evaporator Assembly
to do is remove the heat it contains. Since heat is the
only thing that expanded the refrigerant from a liq-
uid to a vapor in the first place, removal of that same
heat will let the vapor condense into a liquid again.
Then we can return the liquid refrigerant to the
evaporator to be used over again.
Actually, the vapor coming out of the evaporator is
very cold. We know the liquid refrigerant boils at
temperatures considerably below freezing and that
the vapors arising from it are only a shade warmer
even though they do contain quantities of heat.
Consequently, we can’t expect to remove heat from
sub- freezing vapors by “cooling” them in air tem-
peratures that usually range between 60 and 100
degrees heat refuses to
flow from a cold object
toward a warmer object.
But with a pump, we can squeeze the heat-laden
vapor into a smaller space. And, when we compress
the vapor, we also concentrate the heat it contains.
In this way, we can make the vapor hotter without
adding any heat. Then we can cool it in compara-
tively warm air.
That is the only responsibility of a compressor in an
air conditioning system (Fig.
9B-15). It is not in-
tended to be a pump just for circulating the refriger-
ant. Rather, its job is to exert pressure for two
reasons. Pressure makes the vapor hot enough to
cool off in warm air. At the same time, the compres-
sor raises the refrigerant’s pressure above the con-
densing point at the temperature of the surrounding
air so it will condense.
As the refrigerant leaves the compressor, it is still a
vapor although it is now quite hot and ready to give
up the heat that is absorbed in the evaporator. One
of the easiest ways to help refrigerant vapor dis-
charge its heat is to send it through a radiator- like
contrivance known as a condenser (Fig. 9B-16).
The condenser really is a very simple device having
no moving parts. It does exactly the same job as the
radiator in a typical steam-heating system. There,
the steam is nothing more than water vapor. In pass-
ing through the radiator, the steam gives up its heat
and condenses back into water.
The same action takes place in an air conditioning
Page 534 of 625
REFRIGERANT COMPONENTS ALL MODELS9B- 2596.15
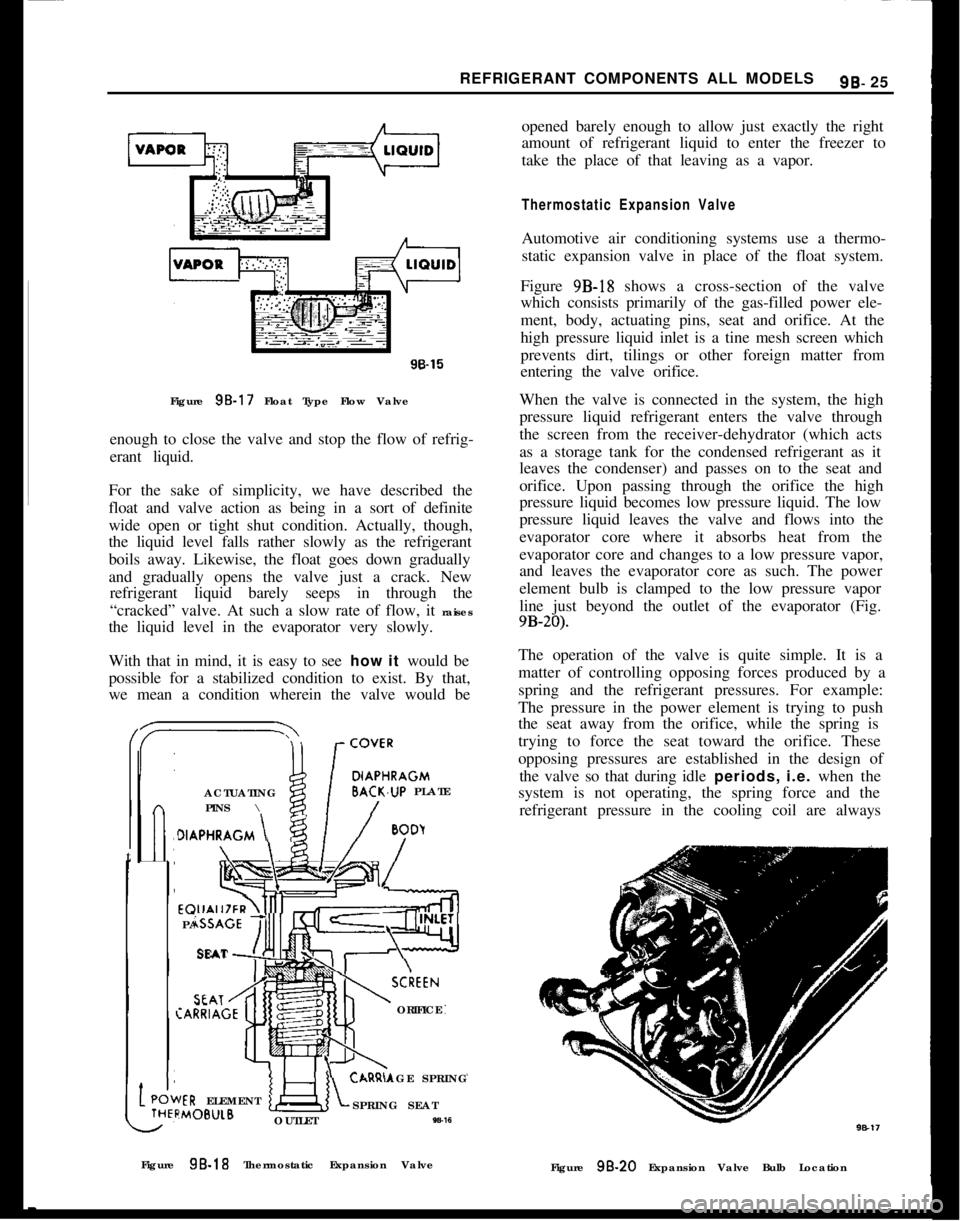
Figure 95.17 Float Type Flow Valve
enough to close the valve and stop the flow of refrig-
erant liquid.
For the sake of simplicity, we have described the
float and valve action as being in a sort of definite
wide open or tight shut condition. Actually, though,
the liquid level falls rather slowly as the refrigerant
boils away. Likewise, the float goes down gradually
and gradually opens the valve just a crack. New
refrigerant liquid barely seeps in through the
“cracked” valve. At such a slow rate of flow, it raises
the liquid level in the evaporator very slowly.
With that in mind, it is easy to see how it would be
possible for a stabilized condition to exist. By that,
we mean a condition wherein the valve would be/
DIAPHRAGMACTUATINGBACK.UP PLATE
PINS \
t
>IAPHRAGM \
/
BoDyEQUALIZER\4]
PASSAGE
‘!!!ISEATSCkEEN:ARRIAGEORIFICE
AGE SPRINGIER ELEMENT:MOB”LBSPRING SEAT
OUTLET
W-16opened barely enough to allow just exactly the right
amount of refrigerant liquid to enter the freezer to
take the place of that leaving as a vapor.
Thermostatic Expansion ValveAutomotive air conditioning systems use a thermo-
static expansion valve in place of the float system.
Figure 9B-18 shows a cross-section of the valve
which consists primarily of the gas-filled power ele-
ment, body, actuating pins, seat and orifice. At the
high pressure liquid inlet is a tine mesh screen which
prevents dirt, tilings or other foreign matter from
entering the valve orifice.
When the valve is connected in the system, the high
pressure liquid refrigerant enters the valve through
the screen from the receiver-dehydrator (which acts
as a storage tank for the condensed refrigerant as it
leaves the condenser) and passes on to the seat and
orifice. Upon passing through the orifice the high
pressure liquid becomes low pressure liquid. The low
pressure liquid leaves the valve and flows into the
evaporator core where it absorbs heat from the
evaporator core and changes to a low pressure vapor,
and leaves the evaporator core as such. The power
element bulb is clamped to the low pressure vapor
line just beyond the outlet of the evaporator (Fig.
9B-20).The operation of the valve is quite simple. It is a
matter of controlling opposing forces produced by a
spring and the refrigerant pressures. For example:
The pressure in the power element is trying to push
the seat away from the orifice, while the spring is
trying to force the seat toward the orifice. These
opposing pressures are established in the design of
the valve so that during idle periods, i.e. when the
system is not operating, the spring force and the
refrigerant pressure in the cooling coil are always
Figure 9B-18 Thermostatic Expansion Valve
Figure
98.20 Expansion Valve Bulb Location
Page 535 of 625
98-26 1973 OPEL SERVICE MANUAL
greater than the opposing pressure in the power ele-
ment. Therefore, the valve remains closed. When the
compressor is started, it will reduce the pressure and
temperature of the refrigerant in the cooling coil to
a point where the vapor pressure in the power ele-
ment becomes the stronger. The seat then moves off
the orifice and liquid starts to flow through the valve
orifice into the cooling coil.
The purpose of the power element is to help deter-
mine the quantity of liquid that is being metered into
the cooling coil. As the temperature of the low pres-
sure line changes at the bulb, the pressure of
the
vapor in the power element changes, resulting in a
change of the position of the seat. For example, if the
cooling coil gets more liquid than is required, the
temperature of the low pressure line is reduced and
the resultant lowering of the bulb temperature
reduces the pressure of the vapor in the power ele-
ment, allowing the seat to move closer to the orifice.
This immediately reduces the amount of liquid leav-
ing the valve. Under normal operation, the power
element provides accurate control of the quantity of
refrigerant to the cooling coil.
To employ our tire pump analogy once more for
clarity, it is the same situation that would exist if you were inflating a tire with a very slow leak. Providing
you pumped the air into the tire as fast as it leaked
out, you would be able to maintain pressure even
though the air would merely be circulating through the tire and leaking out through the puncture.
To Sum Up
So far, we’ve discussed only what each unit in an air
conditioning system does. We’ve learned that the
evaporator is the unit in which liquid refrigerant
soaks up heat from the air, the compressor is a pump
for squeezing this heat out of the vapor, the con-
denser is a radiator for getting rid of the heat, and the
thermostatic expansion valve is a device for regulat-
ing the pressure on the refrigerant. Now, let’s
find
out how the temperature of the cooled air is con-
trolled.
METHOD OF TEMPERATURE CONTROL
To achieve temperature control, the compressor is
run intermittently, automatically turning on and off
as necessary to maintain proper temperature.
Thermostatic Switch
The compressor can be started and stopped au-
tomatically through the use of an electro-magnetic
clutch and a thermostat affected by variations of temperature.
The job is usually done by a gas bulb thermostat (Fig.
9B-21).
Figure 9B-21 Thermostatic Switch Schematic
With the gas bulb type of thermostat, a highly expan-
sive gas is sealed into a metallic bulb which is located
in the air stream as it leaves the evaporator. A small
tube leads from the bulb to a bellows operated switch. As air temperature rises, the gas inside the
bulb expands, travels through the tube to the bellows
and closes the electrical switch that engages the com-
pressor clutch.
Of course, as soon as the compressor starts running,
the temperature begins to go down. As the air being
cooled gets colder, the gas in the thermostat bulb
begins to reduce the pressure on the switch bellows.
This
Ilips “off’ the switch and disengages the com-
pressor clutch.
REFRIGERANTS
No matter how scientifically refrigerating machinery
is built or how
efftciently it runs, it alone cannot
remove heat. The only thing that carries heat out of
a refrigerator cabinet or an automobile is the sub-
stance we call the refrigerant.
There are many refrigerants known to man. In fact,
any liquid that can boil at temperatures somewhere
near the freezing point of water can be used.
But a boiling point below the temperature at which
ice forms is not the only thing that makes a good
refrigerant. A refrigerant should also be non-
poiso-
nowand non-explosive to be safe. Besides that, we
want a refrigerant that is non-corrosive and one that
will mix with oil.
Since Nature did not provide an ideal refrigerant,
chemists went to work to see if they could do any
better. They did! But it wasn’t as simple as that.
At first, they tried to improve existing natural refrig-
erants. But after exploring innumerable trails along
Page 536 of 625

REFRIGERANT COMPONENTS ALL MODELS99.27that line, they still hadn’t gotten anywhere. So, they
started from scratch and juggled molecules around
to make an entirely new refrigerant. Eventually they
succeeded by remodeling the molecules in carbon
tetrachloride. This is the same fluid that is used in
fire extinguishers and dry-cleaners’ solvents.
From this fluid, the chemists removed two chlorine
atoms and replaced them with two fluorine atoms.
This newly-formed fluid carried the technical chemi-
cal name of dichlorodifluoromethane. Today, we
know it as Refrigerant-12 or R-12.
Fluorine is an extremely temperamental substance.
Under most conditions it is toxic and highly corro-
sive, and after is is manufactured, it has to be stored
in special containers because it will eat through glass
and will dissolve most metals in short order.
Despite its rambunctious character though, fluorine
is completely tamed when it is combined with the
other substances that go to make up the refrigerant.
Each is non-toxic, non-inflammable, non-explosive,
and non- poisonous; however, breathing large quan-
tities of R-12 should be avoided.
Pressure. Temperature Relationship of R-12A definite pressure and temperature relationship ex-
ists in the case of liquid refrigerants and their satu-
rated vapors. Increasing the temperature of a
substance causes it to expand. When the substance is
confined in a closed container, the increase in tem-
perature will be accompanied by an increase in pres-
sure, even though no mechanical device was used.
For every temperature, there will be a corresponding
pressure within the container of refrigerant. A table
of the temperature-pressure relationship of R-12 is
presented below. Pressures are indicated in gauge
pressure, either positive pressure (above atmos-
pheric) m pounds or negative pressure (below atmos-
pheric) in inches of vacuum.
“F-40
-35
i#Pressure
11.0*
8.3*
“F
50
50#Pressure
46.1
52.0
-30~
5.5*6057.7
-252.3*6s67 7__.
-200.6
io70.1
-152.4
76.9
-104.584.1
1;6.8 9.2tz99.6 91.71;
11.8 14.712116.9 108.1
1517.7105126.2
2021.1110136.0
2524.6115146.5
3028.5120157.1
;:
30.1
125167.5
32.6
131)179n
4037.0
4541.7*Inches of Vacuum.-. _.-
1402045
150232.0Thus if a gauge is attached to a container of R- 12 and
the room temperature is 70 degrees, the gauge will
register 70 psi pressure; in a 100 degrees room the
pressure will be 117
ps~
AIR CONDITIONINGBecause air conditioning has always been very
closely allied with mechanical refrigeration, most of
us are apt to think of it only as a process for cooling
room air.
But true air conditioning goes beyond the mere cool-
ing of the air. It controls the humidity, cleanliness,
and circulation of the air as well.
Whenever it gets warm and muggy in the summer-
time, someone is almost sure to say, “It’s not the heat
it’s the humidity.” But that is only partly right.
Actually it is a combination of the two that makes us
feel so warm temperature alone is not the only
thing that makes us uncomfortable.
Humidity is nothing more nor less that the moisture
content of the air. To a certain extent, it is tied in
with the temperature of the air. Warm air will hold
more moisture than will cold air. When air contains
all the moisture it can hold, we say it is saturated,
and the relative humidity is 100 percent. If the air
contains only half as much water as it could possibly
hold at any given temperature, we say that the rela-
tive humidity is 50 percent. If it contains only a fifth
of its maximum capacity, we say that the relative
humidity is 20 percent and so on. This amount
of water vapor, or relative humidity, affects the way
we perspire on hot days.
Nature has equipped our bodies with a network of
sweat glands that carry perspiration to the skin
sur-faces. Normally, this perspiration evaporates and, in
doing so, absorbs heat just like a refrigerant absorbs
heat when it is vaporized in a freezer. Most of the
heat thus absorbed is drawn from our bodies, giving
us a sensation of coolness. A drop of alcohol on the
back of your hand will demonstrate this principle
very convincingly. Because it is highly volatile, al-
cohol will evaporate very rapidly and absorb quite a
bit of heat in doing so, thereby making the spot on
your hand feel unusually cool.
The ease and rapidity with which evaporation takes
place, whether it be alcohol or perspiration, governs
our sensation of coolness and to a certain extent,
independently of the temperature. Of even more im-
portance, the ease and rapidity of the evaporation are
directly affected by the relative humidity or com-
parative dampness of the air. When the air is dry,
perspiration will evaporate quite readily. But when
the air contains a lot of moisture, perspiration will
evaporate more slowly; consequently less heat is car-
ried away from our body.
Page 537 of 625
9B-28 1973 OPEL SERVICE MANUAL
Thus, from the standpoint of comfort, complete air
conditioning should control the relative humidity of
the air as well as its temperature.
By reducing the humidity, we sometimes can be just
as “cool” in a higher room temperature than other-
wise would be comfortable. Laboratory tests have
shown that the average person will feel just as cool
in a temperature of 79 degrees when the relative
humidity is down around 30 percent as he will in a
cooler temperature of 72 degrees with a high relative
humidity of 90 percent.
There are practical limits though within which wemust stay when it comes to juggling humidity. For
human comfort, we can’t go much below a relative
humidity of 30 percent because anything lower than
that would cause an unpleasant and unhealthy dry-
ness in the throat and nasal passages.
Summertime temperatures of 85 degrees sometimes
bring with them relative humidities around 75 to 80
percent. Some coastal cities have relative humidities
averaging as high as 87 percent. To gain maximum
human comfort, an air conditioning system should
cool the air down and reduce the humidity to com-
fortable limits.
The cooling job usually is done just as it is in a
refrigerator. A compressor sends refrigerant through
a chilling unit where it absorbs heat. The heat is
drawn out of the air which circulates through the
chilling unit. Along with the cooling job it does, the
evaporator unit also removes much of the moisture
from the air. Everyone is familiar with the sight of
thick frost on the freezer of a refrigerator. That frost
is simply frozen moisture that has come out of the
air.
Figure 99.22 Condensation
The evaporator unit in an air-conditioning system
does the same thing with this one exception. Becauseits temperature is above the freezing point, the mois-
ture does not collect in the form of ice or frost.
Instead, the moisture remains fluid and drips off the
chilling unit. This action is similar to what occurs on
the cool bathroom mirror when a hot shower is
turned on (Fig. 9B-22). A further advantage of airconditioning is that dust and pollen particles are
trapped by the wet surfaces of
.the evaporator core
and then drained off with the condensed moisture.
This provides very clean, pure air for breathing, and
is of great benefit to those who suffer from asthma
or ahergies such as hay fever.
Basic Refrigeration CycleLet’s review the basic refrigeration cycle. Keep this
basic cycle in mind because knowledge of the cycle,
knowledge of the particular system you are working
on and proper use of the gauges will permit quick,
accurate diagnosis of problems as they arise.
Any refrigeration system takes advantage of the
principles just described. The air conditioning sys-
tem illustrated in Fig. 9B-23 contains
five basic parts;
a compressor, a condenser, a receiver, an expansion
valve and an evaporator. Assuming R-12 as our re-
frigerant, let us follow through the refrigeration cy-
cle.Refrigerant gas under low pressure is drawn into the
compressor where it is compressed to a high pres-
sure. During compression, the refrigerant gas is
heated. When sufficient pressure is built up, the hot
gas passes into the condenser where it cools by giving
off heat to the air passing over the condenser sur-
faces.As the refrigerant gas cools, it condenses into a liquid
at high pressure and accumulates in the receiver. The
high pressure liquid refrigerant passes to the expan-
sion valve at the entrance to the evaporator. At the
valve orifice the pressure is lowered and the refriger-
ant enters the evaporator core as a low pressure liq-
uid. When the refrigerant is exposed to the lower
evaporator pressure, it begins to boil and is changed
to a vapor state. As the refrigerant passes through
the evaporator, it continues to boil by absorbing heat
from the air passing over the evaporator surfaces
until it is completely vaporized. From the evaporator
the cool low pressure refrigerant gas is drawn back
to the compressor and the cycle repeated.
Thus the air passing over the evaporator surfaces is
cooled simply by giving up heat to the refrigerant
during the boiling process.
CHEMICAL INSTABILITY AND REFRIGERATING
SYSTEM FAILURESA sealed refrigerating system is a complex physical-
chemical combination which is designed for stability
Page 541 of 625

98-32 1973 OPEL SERVICE MANUAL
inserting it in the connection. Another precaution -inspect the fitting for burrs which can cut the
“0”ring.
Restrictions
Restrictions may be due to powdered desiccant or
dirt and foreign matter. This may result in starved
evaporator and loss of cooling, or a seized compres-
SOT.When the amount of moisture in a system sufti-
ciently exceeds the capacity of the desiccant, it can
break down the desiccant and cause it to powder.
The powder passes through the dehydrator screen
with the refrigerant liquid and is carried to the ex-
pansion valve screen. While some of it may pass
through the valve screen into the evaporator, it may
quickly build up to cause a restriction.
Due to the fact that sufftcient oil can not be returned
to the compressor, it may seize.
Dirt
Dirt, which is any foreign material, may come from
cleaner residues, cutting, machining, or preserving
oils, metal dust or chips, lint or dust, loose rust,
soldering or brazing fluxes, paint or loose oxide
scale. These can also cause seized bearings by abra-
sion or wedging, discharge and expansion valve fail-
ure, decomposition of refrigerant and oil, or
corrosion of metal parts.
CorrosionCorrosion and its by-products can restrict valve and
drier screens, rough bearing surfaces or rapid fatigu-
ing of discharge reeds. This can result in high tem-
perature and pressure, decomposition or leaks. In
any event, this means a wrecked compressor.
From this, we can see the vicious circle that can be
produced in a refrigerating system to cause its fail-
ure. Corrosion can be the indirect cause of leaks, and
leaks can be the direct cause of corrosion. We can
also see the important role we as servicemen play in
maintaining chemical stability.
The major cause of corrosion is moisture.
Moisture
Moisture is the greatest enemy of refrigerating sys-
tems. Combined with metal, it produces oxide, Iron
Hydroxide and Aluminum Hydroxide. Combined
with R-12 it produces Carbonic acid, Hydrochloric
acid, and Hydrofluoric acid. Moisture can also cause
freeze-up of expansion valve and powdered desic-
cant.Although high temperature and dirt are responsible
for many difficulties in refrigerating systems, in most
instances it is the presence of moisture in the system
that accelerates these conditions. It can be said,themfore, that moisture is the greatest enemy of all.
The acids that it produces, in combination with both
the metals and the refrigerant, cause damaging
COT-
rosion. While the corrosion may not form as rapidly
with R-12 as with some other refrigerants, the even-
tual formation is as damaging.
If the operating pressure and temperature in the
evaporator is reduced to the freezing point, moisture
in the refrigerant can collect at the orifice of the
expansion valve and freeze. This temporarily re-
stricts the flow of liquid causing erratic cooling.
As previously mentioned, moisture in excess of the
desiccant’s capacity can cause it to powder.
YOU SHOULD KNOW AND REMEMBER..That the inside of the refrigerat,ion system is com-
pletely sealed from the outside world. And if that
seal remains broken at any point
- the system will
soon be destroyed. That complete and positive seal-
ing of the entire system is vitally important and that
this sealed condition is absolutely necessary to retain
the chemicals and keep them in a pure and proper
condition.
That all parts of the refrigeration system are under
pressure at all times, whether operating or idle, and
that any leakage. points are continuously losing re-
frigerant and oil.
That the leakage of refrigerant can be so silent that
the complete charge may be lost without warning.
That refrigerant gas is heavier than air and will rap-
idly drop to the floor as it flows from a point of
leakage.
That the pressure in the system may momentarily
become as high as 400 lbs. per square inch, and that
under such pressure the molecules of refrigerant are
forced out through the smallest opening or pore.
That the compressor is continually giving up some
lubricating oil to the circulating refrigerant and de-
pends upon oil in the returning refrigerant for con-
tinuous replenishment. Any stoppage or major loss
of refrigerant will therefore be fatal to the compres-
SOT.That the extreme internal dryness of a properly proc-
essed system is a truly desert condition, with the
drying material in the receiver holding tightly on to
the tiny droplets of residual moisture.