change time OPEL GT-R 1973 User Guide
[x] Cancel search | Manufacturer: OPEL, Model Year: 1973, Model line: GT-R, Model: OPEL GT-R 1973Pages: 625, PDF Size: 17.22 MB
Page 279 of 625

58.201973 OPEL SERVICE MANUAL
half, and blow out pistons, carefully regulating air
flow. When removing pistons, proceed with extreme
caution and always keep the fingers ofthe hand hold-
ing the brake caliper away from the piston.
Figure 55.33 Removing Caliper Rim Half Piston
Figure 58-34 Removing Caliper Mounting Half Piston
4. Pry rubber fluid seals out of the annular grooves
in the caliper half bores. See Figure
5B-35.5. Check all parts of the brake caliper for wear. If the
caliper half bores are scored or rusted, use a new
complete brake caliper and friction pads. Small, light
rust spots in the caliper half bores or on the pistons
can be removed with fine emery cloth. If pistons are
damaged, even though the caliper half bores are inFigure 58-35 Removing Rubber Fluid Seal From
Caliper Boresgood condition, the piston must be replaced. The
rubber fluid seals and rubber seals with
clapp rings
for the pistons are to be replaced every time repair
work is carried out on the brake caliper.
6. Thoroughly clean all reusable parts
- complete
brake caliper and pistons
- with denatured alcohol
and dry with compressed air. Prior to cleaning, screw
bleeder valve out of caliper.
7. Lightly coat new rubber fluid seals with brake
fluid and insert fluid seals into grooves of brake
caliper bores.
8. Place brake caliper into vise to install pistons.
After installing one piston, change position of brake
caliper in vise to install second piston. The piston to
friction pad spacer plates should be used as a gauge
to locate relieved edge of piston at 20 degrees to
horizontal during piston installation. See Steps
9-IO-
11-12.9. Place caliper mounting half in vise and coat its
bore and piston lightly with brake fluid. Then push
piston, with hollow end towards brake disc, into the
caliper bore. Turn piston so that the relieved edge
faces downwards at an angle of 20 degrees and facing
in brake disc direction. The guide surface in the
caliper half recess at the brake pipe connection side,
will properly align the piston. Push piston into
caliper bore up to the stop.
10. Change position of brake caliper and install sec-
ond piston in the same manner.
11. Install new rubber seals with clamp rings. Make
sure that the rubber seals are properly seated on the
Page 306 of 625
ENGINE MECHANICAL AND MOUNTS6A- 15New inlet valves must not be refaced or lapped with
grinding compound.The correct angle for the intake
and exhaust valve head is 44 degrees.10. Install cylinder head.
11. Adjust valve clearance. See MAINTENANCE
AND ADJUSTMENTS.
7. Inspect valve guides. Worn or pitted guides can be
reamed to accept valves with oversize stems. Over-
size valves are occasionally used in production.
Oversize valves are marked
’ 1 u “2” or “A” and are
stamped into the valve stem end and also stamped
near spark plug hole. See Figure 6A-22.
Replacing Rocker Arm Studs1. When replacing rocker arm studs become
neces-
sary, remove air cleaner, rocker arm cover and
rocker arm.
8. Reseat valve seats in cylinder head in the following
sequence:
Intake
NOTE:The rocker arm studs are screwed into the
cylinder head. A tapered part of the stem serves to
a void stud loosening.With 45 degrees cutter, remove burnt structure until
a metallic bright seat is obtained. Lightly coat valve
head with red lead, insert it into guide and turn it
under light pressure several times back and forth.
Thereby a contact pattern is obtained and the seat
width can be measured. If valve does not seat per-
fectly all around, lightly recut valve seat to the estab-
lished seat width of
,049” - .059” with 30 degrees
correction cutter.
ExhaustThe directions for reconditioning intake valve seats
apply in principle also to exhaust valve seat recondi-
tioning with the exception that the valve seat width
should be
.063-,073 in. and different cutters are em-
ployed.
NOTE:
: OTse new valve seals whenever
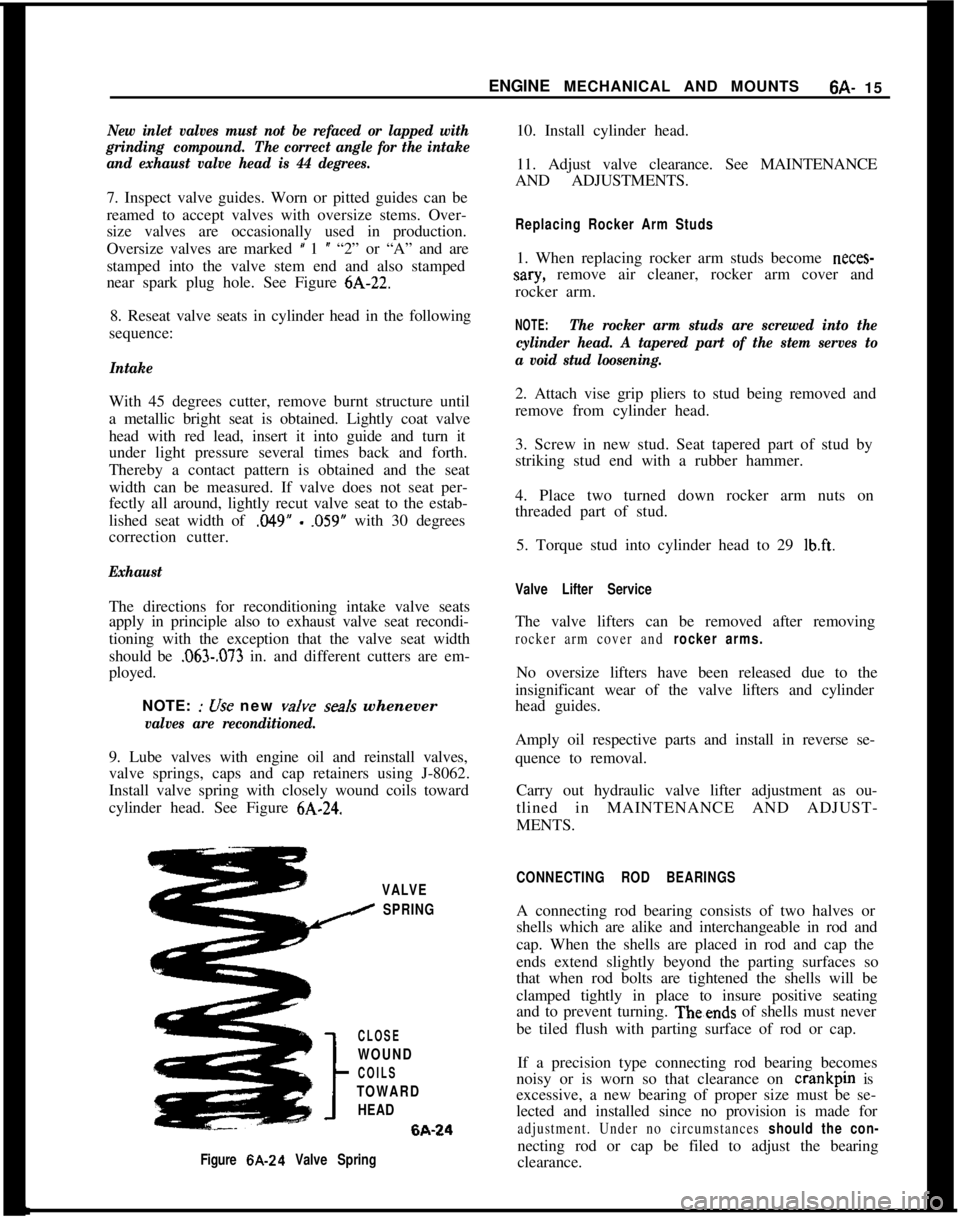
valves are reconditioned.9. Lube valves with engine oil and reinstall valves,
valve springs, caps and cap retainers using J-8062.
Install valve spring with closely wound coils toward
cylinder head. See Figure
6A-24.2. Attach vise grip pliers to stud being removed and
remove from cylinder head.
3. Screw in new stud. Seat tapered part of stud by
striking stud end with a rubber hammer.
4. Place two turned down rocker arm nuts on
threaded part of stud.
5. Torque stud into cylinder head to 29
lb.ft.
Valve Lifter ServiceThe valve lifters can be removed after removing
rocker arm cover and rocker arms.No oversize lifters have been released due to the
insignificant wear of the valve lifters and cylinder
head guides.
Amply oil respective parts and install in reverse se-
quence to removal.
Carry out hydraulic valve lifter adjustment as ou-
tlined in MAINTENANCE AND ADJUST-
MENTS.
VALVE
I SPRING
CLOSE
WOUND
COILS
TOWARD
HEAD6A-24
Figure 6A-24 Valve SpringCONNECTING ROD BEARINGSA connecting rod bearing consists of two halves or
shells which are alike and interchangeable in rod and
cap. When the shells are placed in rod and cap the
ends extend slightly beyond the parting surfaces so
that when rod bolts are tightened the shells will be
clamped tightly in place to insure positive seating
and to prevent turning. Theends of shells must never
be tiled flush with parting surface of rod or cap.
If a precision type connecting rod bearing becomes
noisy or is worn so that clearance on crankpin is
excessive, a new bearing of proper size must be se-
lected and installed since no provision is made for
adjustment. Under no circumstances should the con-necting rod or cap be filed to adjust the bearing
clearance.
Page 307 of 625
6A- 161973 OPEL SERVICE MANUALInspection of Connecting Rod Bearings and
Crankshaft JournalsRemove oil pan.
After removal of oil pan, disconnect two connecting
rods at a time from crankshaft and inspect the bear-
ings and crankpin journals. While,tuming crankshaft
it is necessary to
t&porarily reconnect the rods to
crankshaft to avoid possibility of damaging the jour-
nals through contact with loose rods.
If connecting rod bearings are chipped or scored they
should be replaced. If bearings
are in good physical
condition check for proper clearance on crankpins as
described under, checking clear$nce and selecting
replacement connecting rod beartngs.
If crankpin journals are scored or ridged, the crank-
shaft must be replaced, or reground for undersize
bearings, to insure satisfactory life of connecting rod
bearings. Slight roughness may be polished out withfine grit polishing cloth thoroughly wetted with en-
gine oil. Burrs may be honed off with a fine oil stone.
Use an outside micrometer to check crankpins for
out- of-round. If crankpins are mpre than
,002” out-
of- round, satisfactory life of new ,bearings cannot be
expected.
Checking Clearance and Selecting Replacement
Connecting Rod BearingsService bearings are furnished in standard size and
several undersizes. The clearance of connecting rod
(and crankshaft) bearings may be checked by use of
Plastigage, Type PG-1 (green), or equivalent, which
is soluble in oil.
1. Remove connecting rod cap with bearing shell.
Wipe off oil from bearing and crankpin journal, also
blow oil out of hole in crankshaft.
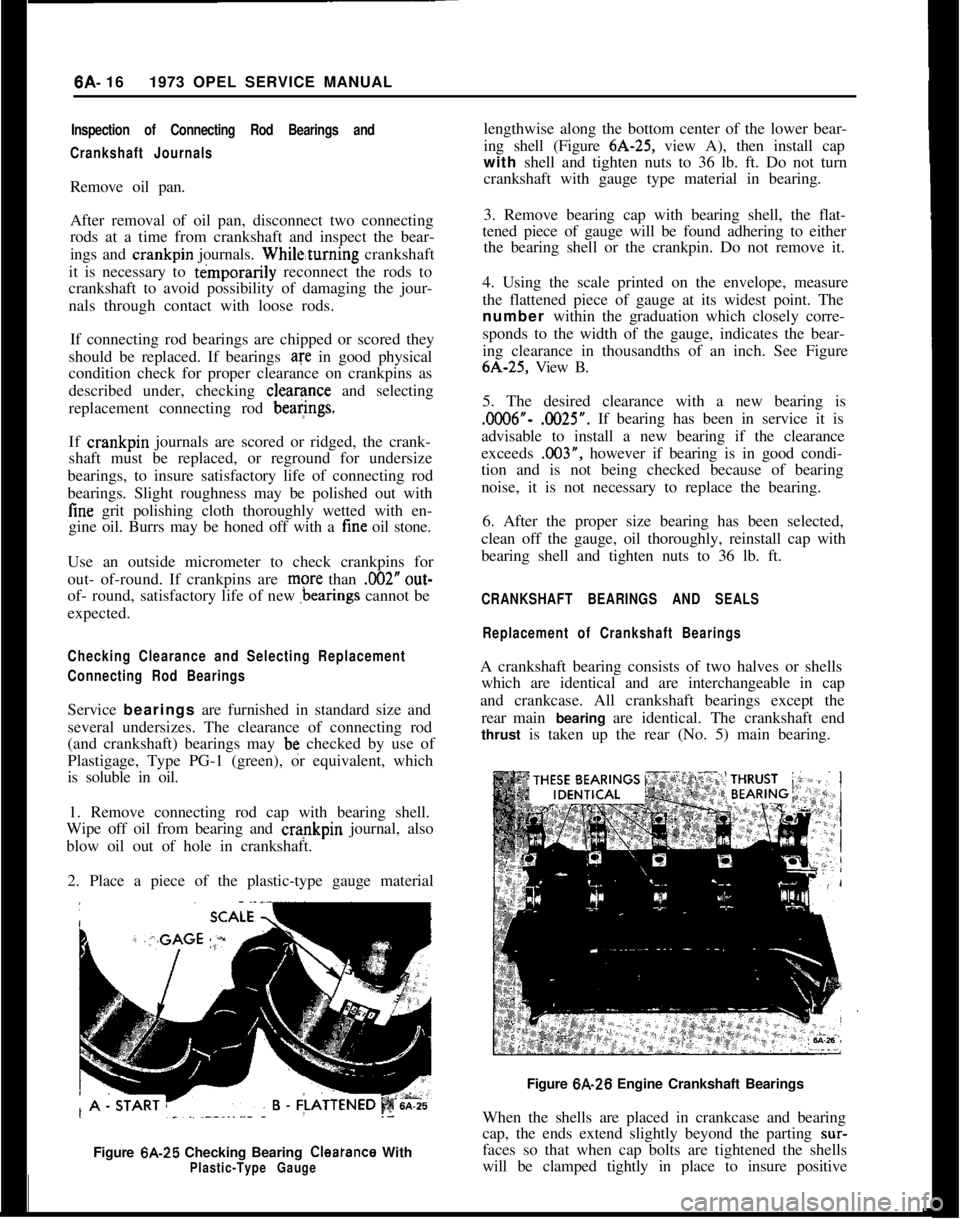
2. Place a piece of the plastic-type gauge material
Figure 6A-25 Checking Bearing
Cleatance WithPlastic-Type Gaugelengthwise along the bottom center of the lower bear-
ing shell (Figure 6A-25, view A), then install cap
with shell and tighten nuts to 36 lb. ft. Do not turn
crankshaft with gauge type material in bearing.
3. Remove bearing cap with bearing shell, the flat-
tened piece of gauge will be found adhering to either
the bearing shell or the crankpin. Do not remove it.
4. Using the scale printed on the envelope, measure
the flattened piece of gauge at its widest point. The
number within the graduation which closely corre-
sponds to the width of the gauge, indicates the bear-
ing clearance in thousandths of an inch. See Figure6A-25, View B.
5. The desired clearance with a new bearing is.0006”- .0025”. If bearing has been in service it is
advisable to install a new bearing if the clearance
exceeds .003”, however if bearing is in good condi-
tion and is not being checked because of bearing
noise, it is not necessary to replace the bearing.
6. After the proper size bearing has been selected,
clean off the gauge, oil thoroughly, reinstall cap with
bearing shell and tighten nuts to 36 lb. ft.
CRANKSHAFT BEARINGS AND SEALS
Replacement of Crankshaft BearingsA crankshaft bearing consists of two halves or shells
which are identical and are interchangeable in cap
and crankcase. All crankshaft bearings except the
rear main bearing are identical. The crankshaft end
thrust is taken up the rear (No. 5) main bearing.
Figure 6A-26 Engine Crankshaft Bearings
When the shells are placed in crankcase and bearing
cap, the ends extend slightly beyond the parting
sur-faces so that when cap bolts are tightened the shells
will be clamped tightly in place to insure positive
Page 336 of 625

CARBURETOR AND THROTTLE LINKAGE6E- 4512345678Sectional View Of 19 US Carburetor (both barrels)
1 PIug(transition channels, secondary barrel)
6 Float chamber
2Carburetor
cover7Idleairpassage
3Vent tube
8Idleairiet4Transit’
,n iet9 Idle air adjusting screw
5 Transition air iet10 Mixture adjusting screw
6E-1Figure 6E-1 Sectional View of Primary and Secondary Barrels
valve gradually opens and the mixture
become+leaner. During this process, the abutment lever
changes position on the fast idle cam, further closingBefore starting a cold engine slowly, depress the ac-celerator pedal three times before engaging the
starter.
the throttle valve until, the engine is at normal oper-
ating temperature, the choke valve is wide open and
the throttle valve is in slow idle position.
Idle and Part Throttle SystemA choke diaphragm is connected to the intermediate
lever of the choke valve spindle through a pull rod.
The vacuum, which develops below the throttle
valve, takes effect on the diaphragm through a
vacuum passage. See Figure
6E-4. As soon as the
engine starts, this vacuum pulls the choke valve
slightly open; the amount of choke valve opening
depends on the amount of vacuum, which depends
on the engine load. Therefore, with a light engine
load, the choke valve will open slightly; with a heavy
engine load, the valve will close slightly to give a
richer mixture as required for this engine load.At engine idle grid during low speed (part throttle)
operation, fuel is drawn from the emulsion tube bore,
controlled by the idle jet and mixed with air entering
through idle air bleeds (Figure 6E-1) and ports in thethrottle body. This mixture is drawn downward to
the three ports near the throttle valve. When the
throttle valve is closed, the mixture is drawn from
the lowest port and mixed with air by-passing the
throttle valve to form the idle mixture.
Turning the idle mixture screw (Figure
6E-1) inward
results in a leaner mixture, and turning it out results
Page 356 of 625
TUNE-UP
ALL MODELS
CONTENTS
Subject
DESCRIPTION AND OPERATION:
Purpose of a Tune-Up. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . , . . . .DIAGNOSIS: (Not Applicable)
MAINTENANCE AND ADJUSTMENTS:
EngineTune-UpMechanicalOperations. . . . . . . . . . . . . . . . . . . .
EngineTune-UpInstrumentChecks. . . . . . . . . . . . . . . . . . . . . . . . . . . .MAJOR REPAIR: (Not Applicable)
SPECIFICATIONS:
Tune-Uo Soecifications and Adjustments
. . . . . . . . . . . . . . . .Page No.6G-65
6G-6566-6766-68
DESCRIPTION AND OPERATION
PURPOSE OF TUNE-UP
The purpose of an engine tune-up is to restore powerand performance that may have been lost through,
loss of adjustment, wear, corrosion, or deterioration
of one or more parts or units. In the normal operat-
ion of an engine, these changes take place gradually
at quite a number of points so that it is seldom advis-able to attempt an improvement in performance by
correcting one or two items only. Time will be savedand more lasting results will be assured by following
a definite and thorough procedure of analysis and
correction of all items affecting power and perform-
ance. Because of Federal laws, limiting exhaust emis-sions, it is even more important that the engines
tune-up is done accurately, using the specifications
listed and the tune-up sticker found in each engine
compartment.
Economical, trouble free operation can better be as-sured if a complete tune-up is performed at first 4
months or
6,ooO miles of operation - then at 12
month or 12,000 mile intervals.
The parts or units which affect power and perform-
ance may be divided, into three groups (1) compres-sion, (2) ignition and (3) carburetion. The tune-up
procedure should cover these groups in the order
given. While the items affecting compression and
ignition may be handled according to individual
preference, correction of items in the carburetiongroup should not be attemplcu
ulllll all items in
compression and ignition have been satisfactorily
corrected.
MAINTENANCE AND ADJUSTMENTS
ENGINE TUNE-UP OPERATIONS
CompressionTo make sure hydrocarbon and carbon monoxide
emissions will be within limits, it is very important
that the adjustments be followed exactly.
The suggested procedure for engine tune-up is as
follows:1. Remove all spark plugs.
2. Position throttle and choke valve in full open posi-tion.
3. Connect jumper wire between distributor terminalof coil and ground on engine to avoid high tension
sparking while cranking engine.
4. Hook up starter remote control cable and turn
ignition switch to “on” position.
5. Firmly insert compression gage in spark plug port.Crank engine to obtain highest possible reading.
Page 529 of 625

98-20 1973 OPEL SERVICE MANUAL
If we were to put a thermometer in the cold drain
water, we would see the temperature gradually creep
upwards. That is to be expected because heat is flow-
ing into the cold water making it warmer. Before
long the water would be as warm as the stored foods.
Then the water could no longer attract heat because
heat will not flow from one warm object to another
equally warm object. Since we no longer can draw
heat out of the foods we no longer are cooling them.
Now, let’s see what happens when we put ice instead
of cold water into the ice-box. This time, we’ll set the
thermometer on top of the ice (Fig. 9B-5). When wefirst look at the thermometer, it reads 32 degrees. A
couple of hours later, we open the ice compartment
door. The ice block is smaller because some of the ice
has already melted away
- but the thermometer still
reads 32 degrees. Again, still later, even more of the
ice has melted, yet the termometer continues to read
32 degrees. So long as any ice remains, no matter
how much of it has melted away, the temperature of
the ice stays right at 32 degrees.
All this time the ice has been soaking up heat, yet it
never gets any warmer no matter how much heat it
draws from the stored food. On the other hand, the
cold drain water got progressively warmer as it
soaked up heat. Why is it the addition of heat will
make water warmer yet won’t raise the temperature
of ice above the 32 degrees mark? If we till one
drinking glass with ice and another with cold water,
and put both glasses in the same room where they
could absorb equal amounts of heat from the room
air, we will find it takes much, much longer for the
ice to melt and reach room temperature than it did
for the water in the other glass to reach the same
temperature. Obviously, most of the heat was being
used to melt the ice. But it was the heat that appar-
ently disappeared or went into hiding because if
couldn’t be located with a thermometer. To best de-
scribe this disappearing heat, scientists turned to
Latin for the right word. They chose the word “la-
tent” which means hidden.
Latent Heat
So latent heat is nothing more nor less than hidden
heat which can’t be found with a thermometer.
What happens to the latent heat? Where does it
disappear to? At first it was thought it was in the
water that melted from the ice. But that wasn’t ex-
actly the right answer because, upon checking water
temperature as it melts from ice, it will be found that
it is only a shade warmer than the ice itself. It is not
nearly warm enough to account for all the heat the
ice had absorbed. The only possible answer is that
the latent heat had been used up to change the ice
from a solid into a liquid.
Many substances can be either a solid, or a liquid, ora gas. It just depends on the temperature whether
water for example was a liquid, or a solid (ice), or gas
(steam) (Fig.
9B-6).Figure 99-6 Temperature Determines State of Water
If we put some water in a tea-kettle, set it over a tire
and watch the thermometer as the water gets hotter
and hotter, the mercury will keep rising until the
water starts to boil. Then the mercury seems to stick
at the 212 degrees mark. If we put more wood on the
fire, despite all the increased heat, the mercury will
not budge above the 212 degree mark (Fig.
9B-7).Figure 98.7 Boiling Water Never Exceeds 2 12
DegreesEven though many housewives won’t believe it, no
matter how large or hot you make the flame, you
can’t make water hotter than 2 12 degrees. As a liquid
changes into a gas, it absorbs abnormally great
amounts of heat without getting any hotter. Here is
another instance where heat disappears.
Now we have two different kinds of latent heat,
which are quite alike. To keep their identities sepa-
rate, the first one is called latent heat of fusion. Since
fusion means the same as melting, it is a good de-
scriptive name. The other kind is called latent heat
of vaporization because‘ that means the same as
evaporation.
It may seem as though we have drifted into a story
Page 530 of 625

REFRIGERANT COMPONENTS ALL MODELSSE- 21
about heat instead of refrigeration. But in doing so,
we have learned how a simple ice-box works. It’s
because the magic of latent heat of fusion gives ice
the ability to soak up quantities of heat without get-
ting any warmer.
Therefore, since it stays cold, it can continue to draw
heat away from stored foods and make them cooler.
The latent heat of vaporization can be an even better
“magnet” because it will soak up even more heat.
Whenever we think of anything boiling, we instinc-
tively think of it being very hot. However, that’s not
true in every case. Just because water
boi1.s at 212
degrees doesn’t mean that all other substances will
boil at the same temperature. Some would have to be
put into a blast furnace to make them bubble and
give off vapor. On the other hand, others will boil
violently while sitting on a block of ice.
And so each substance has its own particular boiling
point temperature. But regardless of whether it is
high or low, they all absorb unusually large quanti-
ties of heat without getting any warmer when they
change from a liquid into a vapor.
Consequently, any liquid that will boil at a tempera-
ture below the freezing point of water, will make ice
cubes and keep vegetables cool in a mechanical re-
frigerator.
Figure
9B-10 Simple R-12 Refrigerator
Refrigerant - 12Refrigerant-12 is used in the air conditioning system
and boils at 21.7 degrees below zero. Maybe that
doesn’t mean very much until we picture a flask of
R-12 sitting at the North Pole boiling away just like
a tea-kettle on a stove. No one would dare pick up
the flask with his bare hands because, even though
boiling, it would be so cold and it would be drawing
heat away from nearby objects so fast that human
flesh would freeze in a very short time. If we were toput a flask of R-12 inside a refrigerator cabinet, it
would boil and draw heat away from everything sur-
rounding it (Fig.
9B-10). So long as any refrigerant
remained in the flask, it would keep on soaking up
heat until the temperature got down to 21.7 degrees
below zero.
Now we can begin to see the similarity between a
boiling tea-kettle and a refrigerator. Ordinarily we
think of the flame pushing heat into the tea-kettle.
Yet, it is just as logical to turn our thinking around
and picture the tea-kettle pulling heat out of the
flame. Both the tea-kettle and the flask of refrigerant
do the same thing they draw in heat to boil
although they do so at different temperature levels.
There also is another similarity between the ice-box
and the mechanical refrigerator. In the ice-box, wa-
ter from melting ice literally carried heat out of the
cabinet. In our simple refrigerator, rising vapors do
the same job.Rdsing
Our R-l 2Water is so cheap that we could afford to throw it
away. But R-12, or any other refrigerant, is too ex-
pensive just to let float away into the atmosphere. If
there was some way to remove the heat from the
vapor and change it back into a liquid, it could be
returned to the flask and used over again (Fig. 9B-
11).There is a way, and that is where we find the biggest
difference between the old ice-box and the modern
refrigerator. We used to put in new ice to replace that
lost by melting. Now we use the same refrigerantover and over again.
Figure 9B-1 1 Re-Using Refrigerant
Page 532 of 625

REFRIGERANT COMPONENTS ALL MODELS96.23Figure 96-l 3 Basic Refrigerant Circuit
we get the heat-laden vapor outside, we can com-
press it with a pump. With enough pressure, we can
squeeze the heat out of “cold” vapor even in a warm
room. An ordinary.radiator will help us get rid of
heat.
By removing the heat, and making the refrigerant
into a liquid, it becomes the same as it was before, So,
we can run another pipe back into the cabinet and
return the refrigerant to the flask to be used over
again.
That is the way most mechanical refrigerators work
today. Now, let’s look at an air conditioning unit to
see how closely it resembles the refrigerator we have
just described.
Basic Air ConditionerWhen we look at an air conditioning unit, we will
always find a set of coils or a tinned radiator core
through which the air to be cooled passes. This is
known as the “evaporator” (Fig.
9B-14). It does the
same job as the flask of refrigerant we
spok.e about
earlier. The refrigerant boils in the evaporator. In
boiling, of course, the refrigerant absorbs heat and
changes into a vapor. By piping this vapor outside
the car we can bodily carry out the heat that caused
its creation.
Once we get vapor out of the evaporator, all we haveFigure 98.14 Evaporator Assembly
to do is remove the heat it contains. Since heat is the
only thing that expanded the refrigerant from a liq-
uid to a vapor in the first place, removal of that same
heat will let the vapor condense into a liquid again.
Then we can return the liquid refrigerant to the
evaporator to be used over again.
Actually, the vapor coming out of the evaporator is
very cold. We know the liquid refrigerant boils at
temperatures considerably below freezing and that
the vapors arising from it are only a shade warmer
even though they do contain quantities of heat.
Consequently, we can’t expect to remove heat from
sub- freezing vapors by “cooling” them in air tem-
peratures that usually range between 60 and 100
degrees heat refuses to
flow from a cold object
toward a warmer object.
But with a pump, we can squeeze the heat-laden
vapor into a smaller space. And, when we compress
the vapor, we also concentrate the heat it contains.
In this way, we can make the vapor hotter without
adding any heat. Then we can cool it in compara-
tively warm air.
That is the only responsibility of a compressor in an
air conditioning system (Fig.
9B-15). It is not in-
tended to be a pump just for circulating the refriger-
ant. Rather, its job is to exert pressure for two
reasons. Pressure makes the vapor hot enough to
cool off in warm air. At the same time, the compres-
sor raises the refrigerant’s pressure above the con-
densing point at the temperature of the surrounding
air so it will condense.
As the refrigerant leaves the compressor, it is still a
vapor although it is now quite hot and ready to give
up the heat that is absorbed in the evaporator. One
of the easiest ways to help refrigerant vapor dis-
charge its heat is to send it through a radiator- like
contrivance known as a condenser (Fig. 9B-16).
The condenser really is a very simple device having
no moving parts. It does exactly the same job as the
radiator in a typical steam-heating system. There,
the steam is nothing more than water vapor. In pass-
ing through the radiator, the steam gives up its heat
and condenses back into water.
The same action takes place in an air conditioning
Page 533 of 625

9B-24 1973 OPEL SERVICE MANUAL
Figure 9B-15 Compressor Assembly - GT Shown
Figure 3B-16 Condenser Assembly
condenser. The refrigerant vapor gives up its heat,
which is quickly and easily radiated into the sur-
rounding air through the large finned surfaces of the
condenser. In giving up its heat, the refrigerant vapor
condenses back into liquid which collects in a pool
at the bottom of the condenser.
As we have said before, when the refrigerant con-
denses into a liquid, it again is ready for boiling in the
evaporator. So, we can run a pipe from the condenser
back to the evaporator.
Main Units of the SystemThese three units then; the evaporator, the compres-
sor, and the condenser are the main working
parts of any typical air conditioning system. We have
the evaporator where the refrigerant boils andchanges into a vapor, absorbing heat as it does so. We
have the pump or compressor to put pressure on the
refrigerant so it can get rid of its heat. And we have
a condenser outside the car body to help discharge
the heat into the surrounding air.
Pressure and FlowThere is one more unit that co-operates with thesethree. It doesn’t do any real work, but it does act as
sort of a traffic officer in controlling the flow of the
refrigerant through the system. To get a better idea
of what this does. let’s first do a li,ttle exoerimentine
with an ordinary’ tire pump.
When we use a
t,ire pump to Sate an automobile
tire, we are creating pressure only because we are
“pushing” against the air already entrapped inside
the tire. If you question this, just try pumping up a
tire that has a large puncture in it. You could pump
all day, and still not be able to build up any pressure.
As fast as you would pump the air in, it would leak
out through the puncture.
Abou~t all you would be
doing would be circulating nice fresh air through the
tire.
1Jnless you have something lo push against - to
block the tlow of air
- you can’t create more than a
mere semblance of pressure.
The same situation holds true in an air conditioning
system. The compressor can pump refrigerant vapor
through the system, but unless it has something to
push against, it cannot build up pressure. All the
compressor would be doing would be to circulate the
vapor without increasing its
pres,sure.Yet we can’t just block the flow through the system
entirely. All we want to do is put pressure on the
refrigerant vapor so it will condense at normal tem-
peratures. What’s more, this
musi: be done some time
after the vapor leaves the evaporator and before it
returns again as a liquid. We can’t have high pressure
in the evaporator because that would slow down the
boiling of the refrigerant and thus penalize the re-
frigerating effect.
Controlling Pressure and FlowPressure and flow can be controlled with a float
valve, or with a pressure-regulating valve. They do
the same job, but in a different way.
Since the float valve type will give us a better idea of
pressure and flow control, let’s look at it first (Fig.
9B-17).It consists simply of a float that rides on the surface
of the liquid refrigerant. As the refrigerant liquid
boils and passes off as a vapor, naturally the liquid
level drops lower and lower. Correspondingly, the
float, because it rides on the surface of the refriger-
ant, also drops lower and lower as the liquid goes
down.By means of a simple system of mechanical linkage,
the downward movement of the float opens a valve
to let refrigerant in. The incoming liquid raises the
fluid level and, of course, the float rides up with it.
When the surface level of the refrigerant liquid re-
aches a desired height, the float: will have risen far
Page 537 of 625
9B-28 1973 OPEL SERVICE MANUAL
Thus, from the standpoint of comfort, complete air
conditioning should control the relative humidity of
the air as well as its temperature.
By reducing the humidity, we sometimes can be just
as “cool” in a higher room temperature than other-
wise would be comfortable. Laboratory tests have
shown that the average person will feel just as cool
in a temperature of 79 degrees when the relative
humidity is down around 30 percent as he will in a
cooler temperature of 72 degrees with a high relative
humidity of 90 percent.
There are practical limits though within which wemust stay when it comes to juggling humidity. For
human comfort, we can’t go much below a relative
humidity of 30 percent because anything lower than
that would cause an unpleasant and unhealthy dry-
ness in the throat and nasal passages.
Summertime temperatures of 85 degrees sometimes
bring with them relative humidities around 75 to 80
percent. Some coastal cities have relative humidities
averaging as high as 87 percent. To gain maximum
human comfort, an air conditioning system should
cool the air down and reduce the humidity to com-
fortable limits.
The cooling job usually is done just as it is in a
refrigerator. A compressor sends refrigerant through
a chilling unit where it absorbs heat. The heat is
drawn out of the air which circulates through the
chilling unit. Along with the cooling job it does, the
evaporator unit also removes much of the moisture
from the air. Everyone is familiar with the sight of
thick frost on the freezer of a refrigerator. That frost
is simply frozen moisture that has come out of the
air.
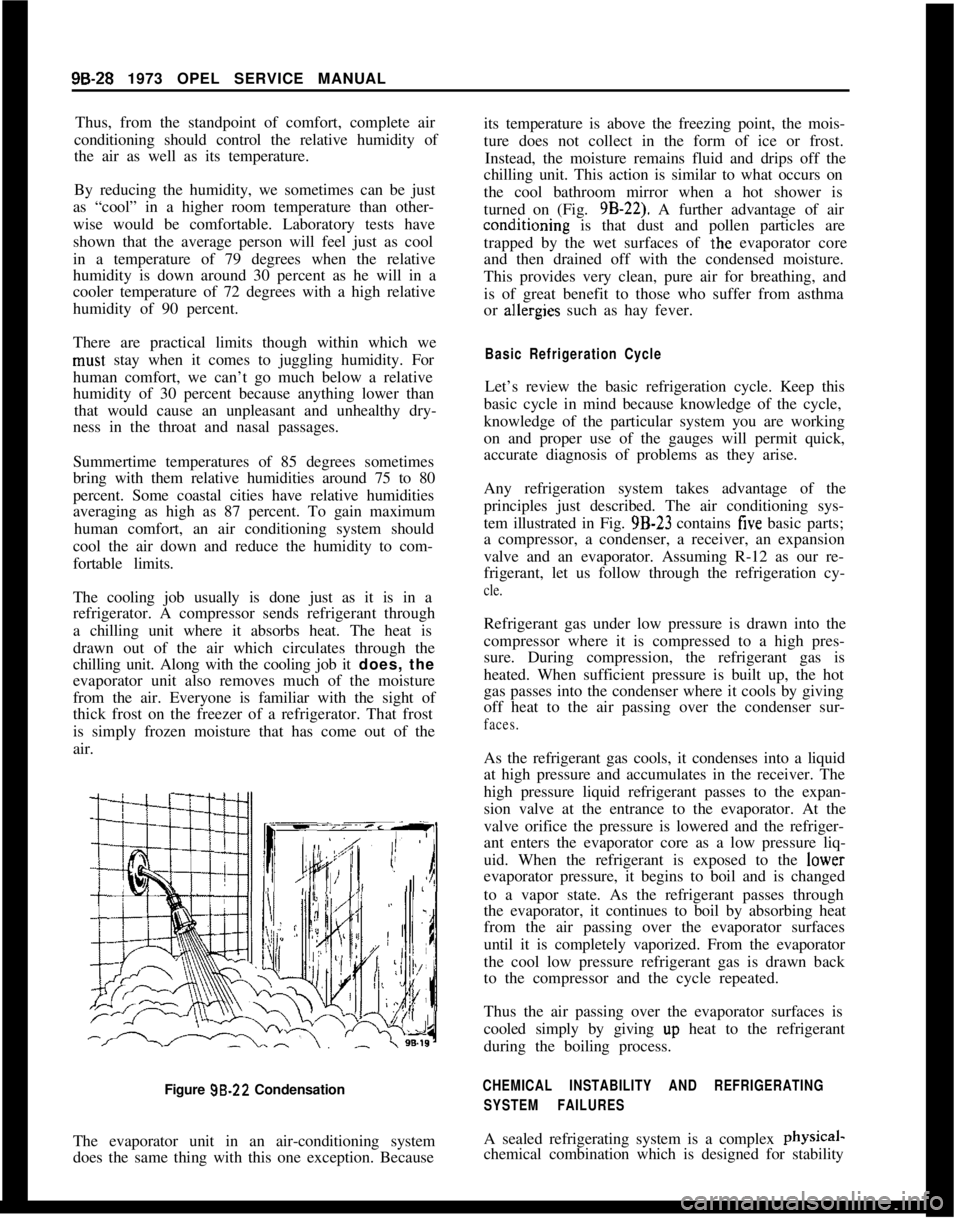
Figure 99.22 Condensation
The evaporator unit in an air-conditioning system
does the same thing with this one exception. Becauseits temperature is above the freezing point, the mois-
ture does not collect in the form of ice or frost.
Instead, the moisture remains fluid and drips off the
chilling unit. This action is similar to what occurs on
the cool bathroom mirror when a hot shower is
turned on (Fig. 9B-22). A further advantage of airconditioning is that dust and pollen particles are
trapped by the wet surfaces of
.the evaporator core
and then drained off with the condensed moisture.
This provides very clean, pure air for breathing, and
is of great benefit to those who suffer from asthma
or ahergies such as hay fever.
Basic Refrigeration CycleLet’s review the basic refrigeration cycle. Keep this
basic cycle in mind because knowledge of the cycle,
knowledge of the particular system you are working
on and proper use of the gauges will permit quick,
accurate diagnosis of problems as they arise.
Any refrigeration system takes advantage of the
principles just described. The air conditioning sys-
tem illustrated in Fig. 9B-23 contains
five basic parts;
a compressor, a condenser, a receiver, an expansion
valve and an evaporator. Assuming R-12 as our re-
frigerant, let us follow through the refrigeration cy-
cle.Refrigerant gas under low pressure is drawn into the
compressor where it is compressed to a high pres-
sure. During compression, the refrigerant gas is
heated. When sufficient pressure is built up, the hot
gas passes into the condenser where it cools by giving
off heat to the air passing over the condenser sur-
faces.As the refrigerant gas cools, it condenses into a liquid
at high pressure and accumulates in the receiver. The
high pressure liquid refrigerant passes to the expan-
sion valve at the entrance to the evaporator. At the
valve orifice the pressure is lowered and the refriger-
ant enters the evaporator core as a low pressure liq-
uid. When the refrigerant is exposed to the lower
evaporator pressure, it begins to boil and is changed
to a vapor state. As the refrigerant passes through
the evaporator, it continues to boil by absorbing heat
from the air passing over the evaporator surfaces
until it is completely vaporized. From the evaporator
the cool low pressure refrigerant gas is drawn back
to the compressor and the cycle repeated.
Thus the air passing over the evaporator surfaces is
cooled simply by giving up heat to the refrigerant
during the boiling process.
CHEMICAL INSTABILITY AND REFRIGERATING
SYSTEM FAILURESA sealed refrigerating system is a complex physical-
chemical combination which is designed for stability